About LARC Robotics
LARC Robotics is developing robotic solutions to render every patient stone-free in a single percutaneous nephrolithotomy (PCNL) procedure.
PCNL is the technique with the highest stone-free rate, and the best chance to have a single-surgery treatment. However, the procedure adoption is still low (around 7%), as establishing an effective fluoroscopy-guided renal access is difficult and increases operative time and radiation exposure. Urologists usually rely on Interventional Radiologists to obtain access, which increases cost and negatively affects scheduling, without obvious outcome improvements. They need a solution that enables them to perform an image-guided renal access for PCNL procedures independently, in a simple and accurate way.
Founded as a spin-off of Interventional Systems, LARC Robotics leverages the Austria-based company’s years of expertise, products, and know-how in the complex interventional radiology landscape to revolutionize the kidney stone market with a surgical robotic platform that empowers urologists to easily gain renal access in PCNL procedures.
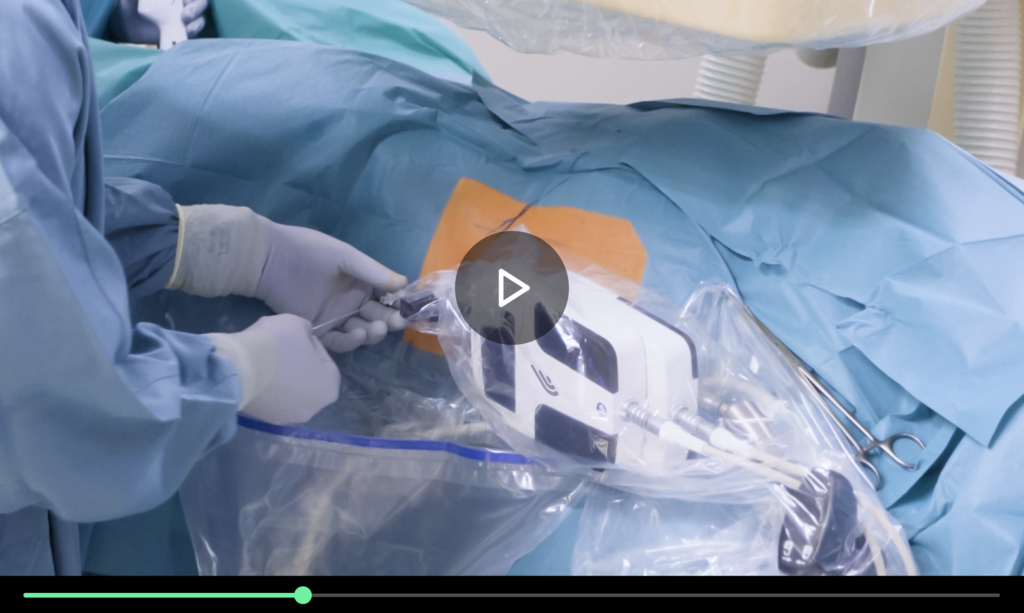
The LARC Robot is a table-mounted miniature device that simplifies renal access through intraoperative planning, targeting under real-time imaging, and precise robotic-assisted needle insertion. The technology is already CE-marked and FDA-cleared for 2D and 3D image-guided procedures.
Clinical validation is already underway, and further development will include supporting 2D-fluoro navigation and ultrasound guidance, a digital platform for surgeons to visualize patient-specific, high-fidelity 3D reconstructions of the diagnostic scans to prepare for the procedure, and a data and analytics module for post-procedural analysis, to find areas of opportunity and performance trends.